![]() When I finished my PhD 15 years ago, the neurosciences defined the main function of brains in terms of processing input to compute output: “brain function is ultimately best understood in terms of input/output transformations and how they are produced” wrote Mike Mauk in 2000 (DOI: 10.1038/76606). Since then, a lot of things have been discovered that make this stimulus-response concept untenable and potentially based largely on laboratory artifacts.
When I finished my PhD 15 years ago, the neurosciences defined the main function of brains in terms of processing input to compute output: “brain function is ultimately best understood in terms of input/output transformations and how they are produced” wrote Mike Mauk in 2000 (DOI: 10.1038/76606). Since then, a lot of things have been discovered that make this stimulus-response concept untenable and potentially based largely on laboratory artifacts.
For instance, it was discovered that the likely ancestral state of behavioral organization is one of probing the environment with ongoing, variable actions first and evaluating sensory feedback later (i.e., the inverse of stimulus response). It was found that even the most rigid and iconic of stimulus-response systems – spinal reflexes – still show rudiments of probing the environment with spontaneous, variable actions and evaluating the sensory consequences later. A recently discovered instance of a so-called ‘rare predator’ phenomenon exemplified that rigid stimulus-response coupling cannot be evolutionary stable:
Kevin Mitchell thus aptly referred to the hypothetical class of animals without unpredictability “lunch” (see his excellent article for a more verbose explanation). In humans, functional magnetic resonance imaging (fMRI) studies over the last decade and a half revealed that the human brain is far from passively waiting for stimuli, but rather constantly produces ongoing, variable activity (the so-called default mode network, DMN, in our resting state), and just shifts this activity over to other networks when we move from rest to task or switch between tasks. Tellingly, the variations in DMN activity account for a large part of our behavioral variability.
As one would expect, this dramatic shift in perspectives from input/output to output/input has led to a slew of recent publications which were not thinkable a mere 15 years ago. For instance, it was reported that rodent brains add variability to sensory input. In Aplysia, it was shown that such variability can be generated by balancing excitatory and inhibitory input, but also that individual neurons (see Fig. 4b) are capable of showing spontaneous variability in their firing patterns, even when they are isolated from the rest of the nervous system. In the most recent annual meeting of the Society for Neuroscience, where I usually only find very few presentations on ongoing activity and how it leads to variability, there now were several posters on exactly this topic, seemingly out of nowhere. The most recent publication is from an animal where the connectome is so dominated by feed-forward connections from sensory to motor neurons, that even today it would be difficult to imagine how the neurobiology underlying behavioral variability could be studied in such an animal, the nematode worm Caenorhabditis elegans: Feedback from Network States Generates Variability in a Probabilistic Olfactory Circuit.
In this paper from the laboratory of Cori Bargmann, Gordus et al. look at a circuit in the C. elegans nervous system which controls reversal behaviors (Fig. 1). The main component of the system is a neuron called AVA. When AVA is active, the animal reverses its course. Sensory input to this neuron is provided by olfactory neuron AWC. For instance, if AWC is stimulated by an attractive odorant, it stops firing, such that AVA also stops firing, making reversals less likely. Two additional neurons are involved in this circuit, AIB and RIM, and the characterization of their role in the circuit was the main result of this publication.
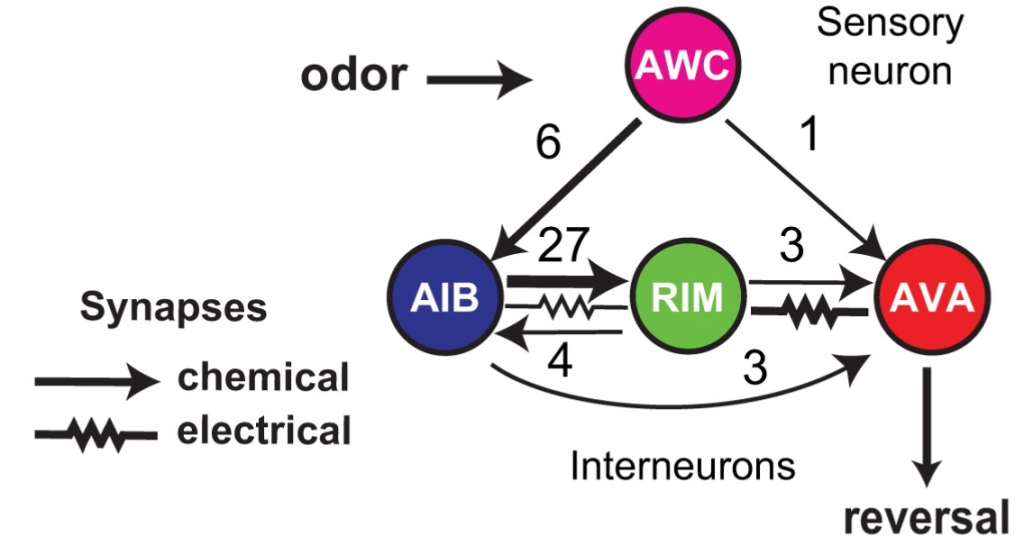
Fig. 1: The C. elegans reversal circuit with the number of electrical and chemical synapses between each network component
The first interesting observation from the circuit connectivity is that there are more connections from the sensory neuron to the AIB interneuron than to the reversal neuron AVA. This would be a major head scratcher if the main function of nervous systems were to relay sensory information to motor centers, but if sensory input merely modulates ongoing activity, even in nematodes, then this doesn’t seem so surprising any more.
Contrary to the idea that a connectome dominated by feed-forward connections from sensory to motor areas implies that it mainly computes motor output from sensory input, also the nervous system of C. elegans is best characterized by constantly changing, ongoing activity, much like all the other nervous systems previously studied in this regard. Even the small circuit studied by Gordus et al. demonstrates that:
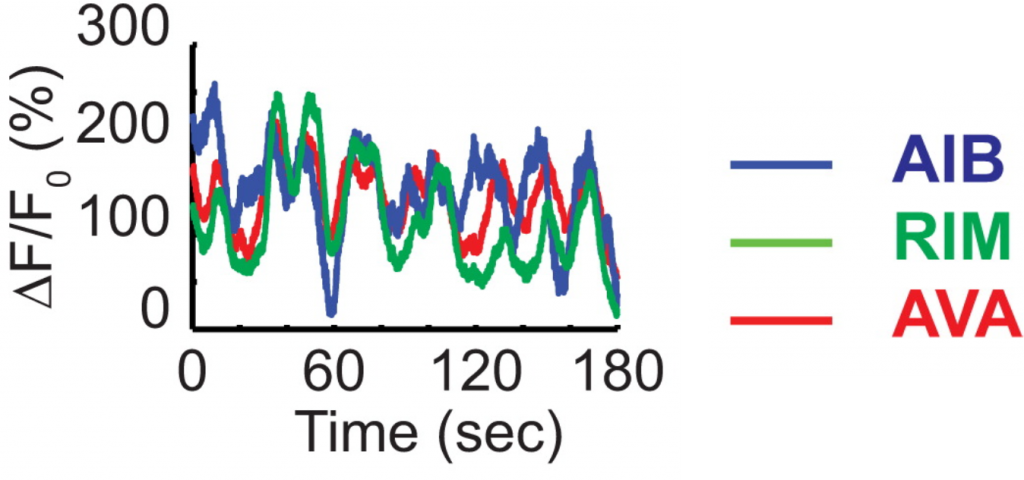
Fig. 2: Even in the absence of sensory stimulation in immobilized animals, the activity in the reversal circuit fluctuates constantly. The Y-axis depicts fluorescence as a measure of calcium levels (i.e., activity) in the neurons.
Interestingly, the neurons exhibit a sort of binary activity state, that for the most part is either on (neuron is active) or off (neuron is inactive):
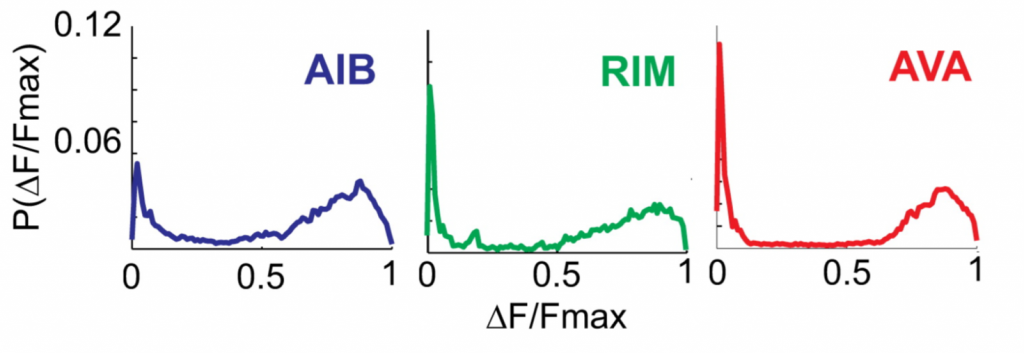
Fig.: 3: The three neurons spend most of their time either in an ‘off’ state or in an ‘on’ state, as can be seen from the probability (P) of fluorescence (F).
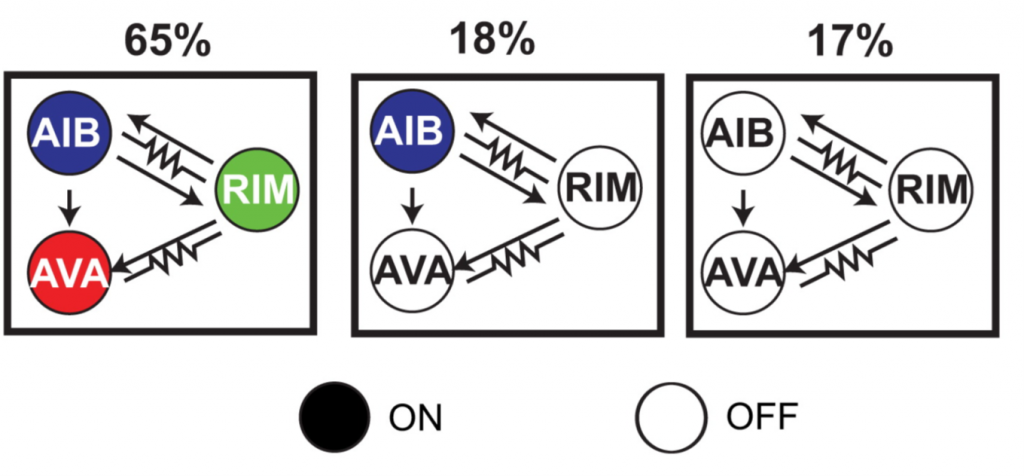
According to this classification, there are three main states (of the eight theoretically possible) the circuit is commonly found in, mainly due to the strong correlation between neurons because of their electrical and chemical coupling: just over 60% of the time the system is in ‘all on’, roughly 20% is ‘all off’ and for the remaining 20% it is in ‘only AIB on’:
By selectively inhibiting each member of the circuit, the authors discovered that the role of AIB and RIM was to increase the variability of the reversal circuit. The input from the olfactory neuron AWC was always very precise and predictable if, e.g., an attractive odor was presented, but the activity of the reversal circuit always varied significantly and this variability was reduced if AIB or RIM were silenced. An example of how variable the response of the circuit is compared to the sensory input without any experimental manipulation of AIB or RIM can be seen in Fig. 5:
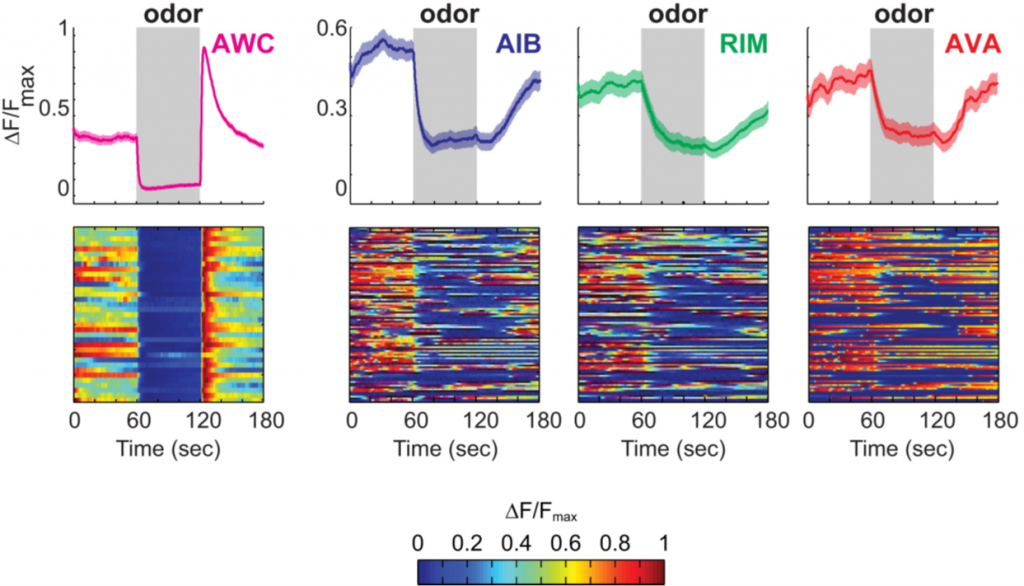
The olfactory neuron AWC responds is drastically more deterministic to an attractive odor than the components of the reversal circuit.
Thus, the authors make an excellent case for RIM and AIB being incorporated into the reversal circuit specifically to inject much needed variability into an otherwise maladaptively deterministic reversal circuit. Surprisingly, even though the feed-forward connections dominate the connectivity also in this little circuit, the variability provided by the feedback connections dominate an adaptive feature of the behavior, its variability. This work adds C. elegans to the elongating list of animals, whose nervous systems are organized such that ongoing activity is modulated by external stimuli. It seems, in such nervous systems, even a numerically small feedback component provides a fundamental contribution to the overall architecture. What does this mean for brains whose anatomy appears to be dominated by feedback loops?
Gordus, A., Pokala, N., Levy, S., Flavell, S., & Bargmann, C. (2015). Feedback from Network States Generates Variability in a Probabilistic Olfactory Circuit Cell DOI: 10.1016/j.cell.2015.02.018













Comments are closed.