How to improve motor learning in Drosophila
- Version
- Download 1897
- File Size 0.00 KB
- File Count 1
- Create Date September 24, 2024
- Last Updated September 24, 2024
How to improve motor learning in Drosophila
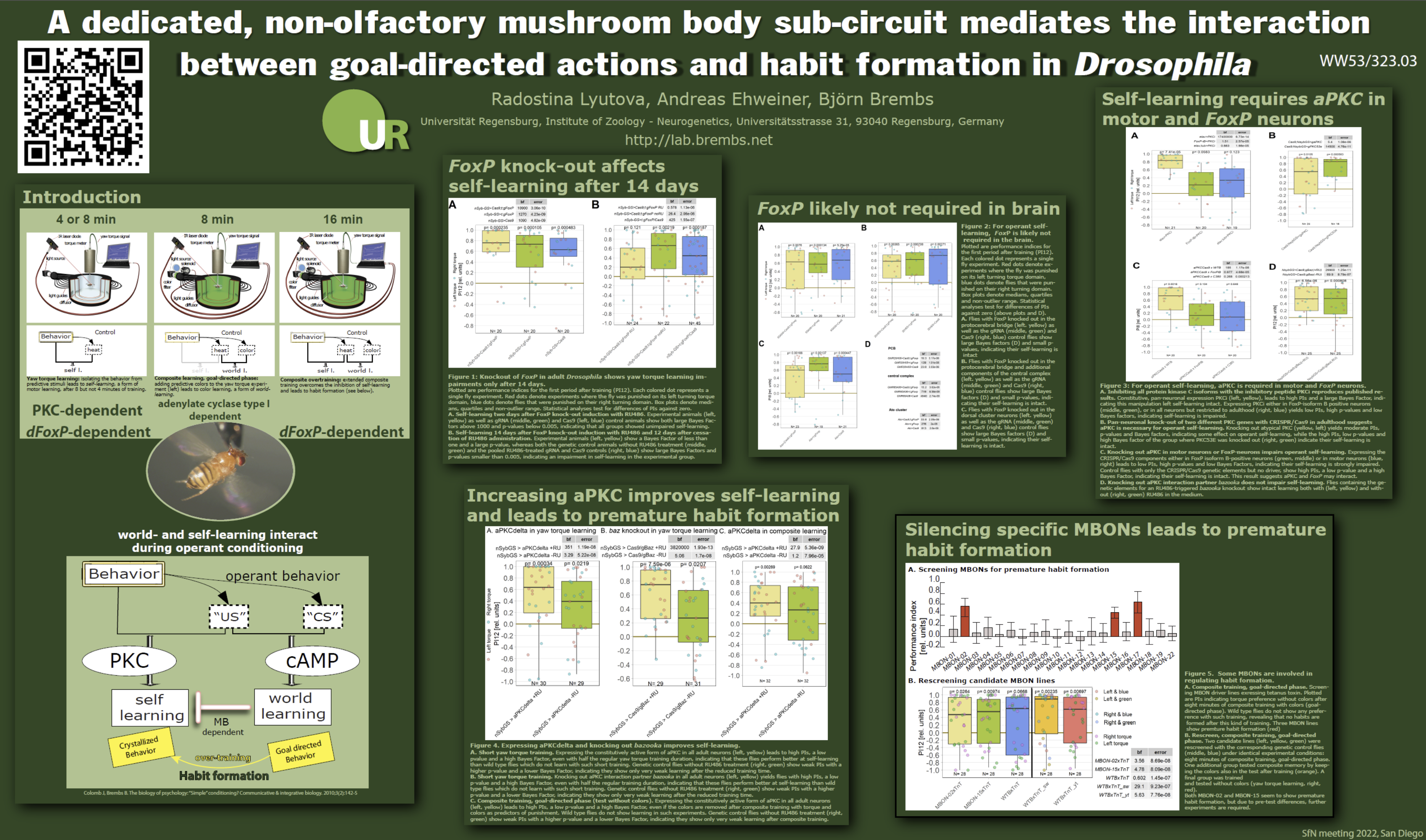
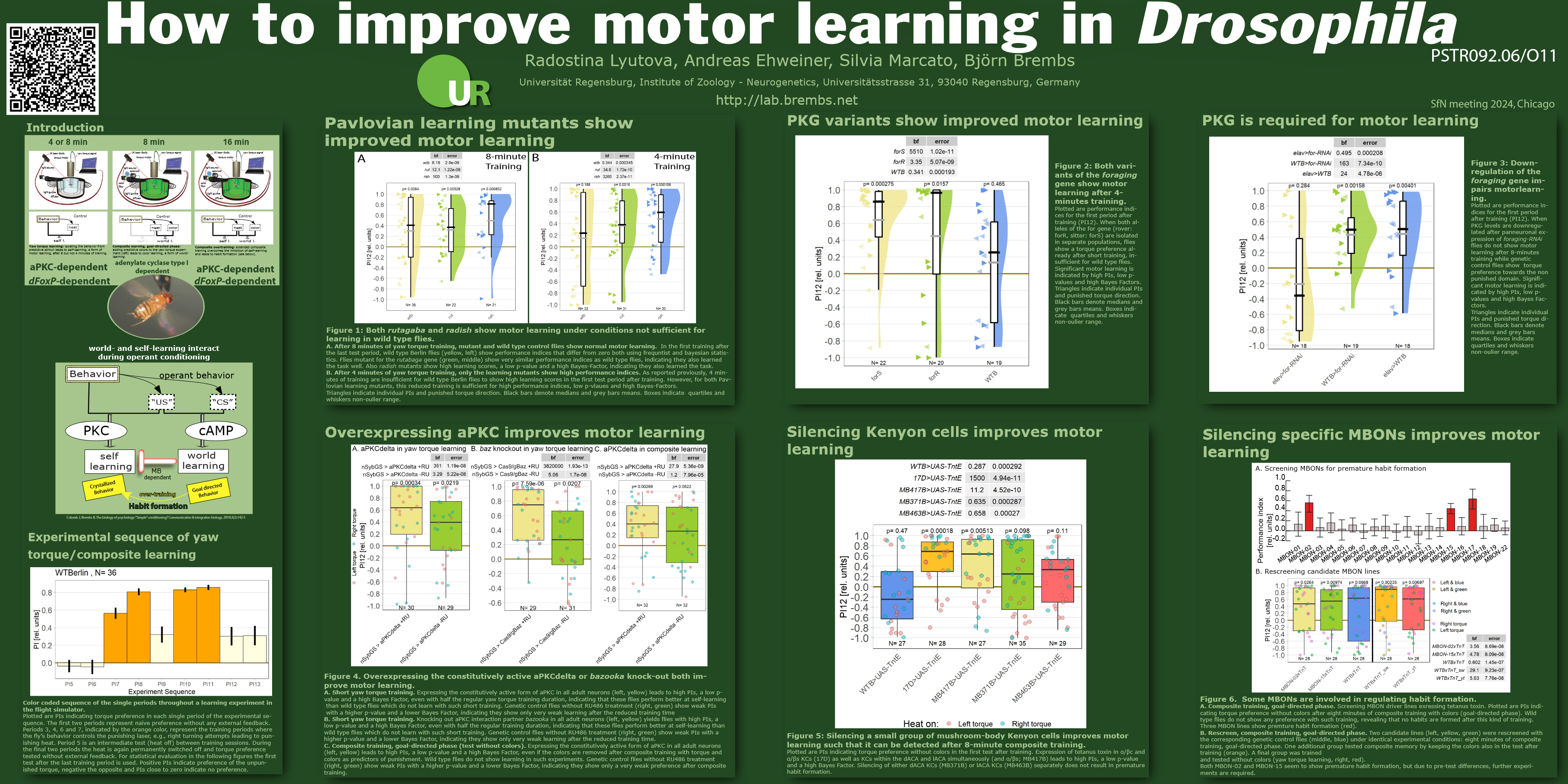
Motor learning, skill-learning or habit formation share conceptual similarities, but it is debated how much biology these processes have in common. There is genetic evidence linking motor learning and habit formation in flies, song-learning in birds and language acquisition in humans to an evolutionary conserved operant self-learning process. We show different biological manipulations of Drosophila that all enhance motor learning. We suggest a world-learning mechanism inhibiting premature habit formation and interfering with self-learning. We identified a neural circuit which actively controls the process of motor learning depending on the animal’s environment. Our results show that mutations in genes involved in classical conditioning, such as rutabaga or radish, enhance motor learning. Overexpression of an operant learning gene, atypical PKC (aPKC) enhances motor learning as well as habit formation. Inhibition of an aPKC interaction partner, bazooka, also enhances motor learning. We will also show data comparing the motor learning efficacy of rover and sitter flies, which carry different variants of the protein kinase G (PKG) gene. We further investigated the effects of PKG RNAi knockdown on motor learning. Further, inhibition of a prominent insect neuropil, the mushroom bodies (MBs), leads to formation of premature habits. We show that this function is mediated via MB output neuron 2 (MBON-02). The anatomy of this neuron indicates that non-olfactory MB Kenyon cells (KCs) of the β2 and β’2-lobes are involved in this enhancement by receiving input within the little-studied lateral (lACA) and dorsal (dACA) accessory calyx regions of the MB. However, our data shows that both the visual (via dACA) and the thermosensory (via lACA) input are not necessary for premature habit formation. We propose a network within the MB circuitry controlling the transition from goal-directed behavior (world-learning) to habits (self-learning). We hypothesize that MBON-02 might be a site of coincident input of sensory stimuli in combination with signal amplification via KC-to-KC feed-forward loops. Given the conserved nature of these learning processes in all bilaterian animals including humans and the role of motor learning in language acquisition, habit formation/addiction and rehabilitation after stroke or spinal cord injury, the diversity of these learning enhancements promises a rich field for the development of medical applications.